Overview
While sugar often takes the blame for Type 2 diabetes, it’s crucial to delve into the role of fats in this complex health puzzle.
A recent study conducted at the University of Geneva (UNIGE) delved into the intricacies by examining the blood profiles of individuals with diabetes, pre-diabetes, or those who underwent partial pancreas removal.
The findings unveiled two significant revelations.
Firstly, the lipid composition in both blood and fatty tissues experiences fluctuations throughout the day, with people with diabetes showcasing altered patterns featuring elevated levels of harmful lipids.
Secondly, a specific type of lipid known as lysoPI demonstrated a remarkable ability to enhance insulin secretion, offering potential solutions for instances where beta cells, responsible for insulin production, face challenges.
Why are these findings significant?
Unravelling the Role of Fats in Type 2 Diabetes
Understanding the role of lipids in the regular and abnormal workings of human metabolism is an evolving narrative, gaining clarity, especially in the context of type 2 diabetes—a prevalent and severe metabolic disorder.
Advanced technologies, such as mass spectrometry, have empowered researchers to assess the levels of numerous lipid varieties concurrently. Each lipid, with its distinctive features, carries the potential for either positive or adverse impacts on our metabolism.
These groundbreaking findings, featured in the journals Cell Reports Medicine and Diabetes, hold significant promise for treating individuals grappling with diabetes.

Charna Dibner and Pierre Maechler, professors in the Department of Surgery and the Department of Cell Physiology and Metabolism at the UNIGE Faculty of Medicine and key contributors to these studies at the Diabetes Faculty Center emphasise the vast potential of identifying the predominant lipids in individuals with type 2 diabetes.
They point to many possibilities, from early detection and prevention to identifying therapeutic targets or offering personalised recommendations.
Deciphering Diabetic Lipid Dynamics
To substantiate their findings, the team thoroughly examined blood profiles from patients across four European countries. Furthermore, they validated some of their discoveries by replicating the study on a mouse disease model.
Professor Charna Dibner’s team, specialising in circadian rhythms in metabolic disorders, embarked on a comprehensive “lipidomic” analysis involving two distinct patient groups. They aimed to delineate the 24-hour cycle profiles of various lipids in blood and adipose tissues.
Remarkably, the team uncovered pronounced disparities in lipid profiles between individuals with type 2 diabetes and those without, particularly during the early morning, and this period exhibited an upsurge in specific harmful lipids.
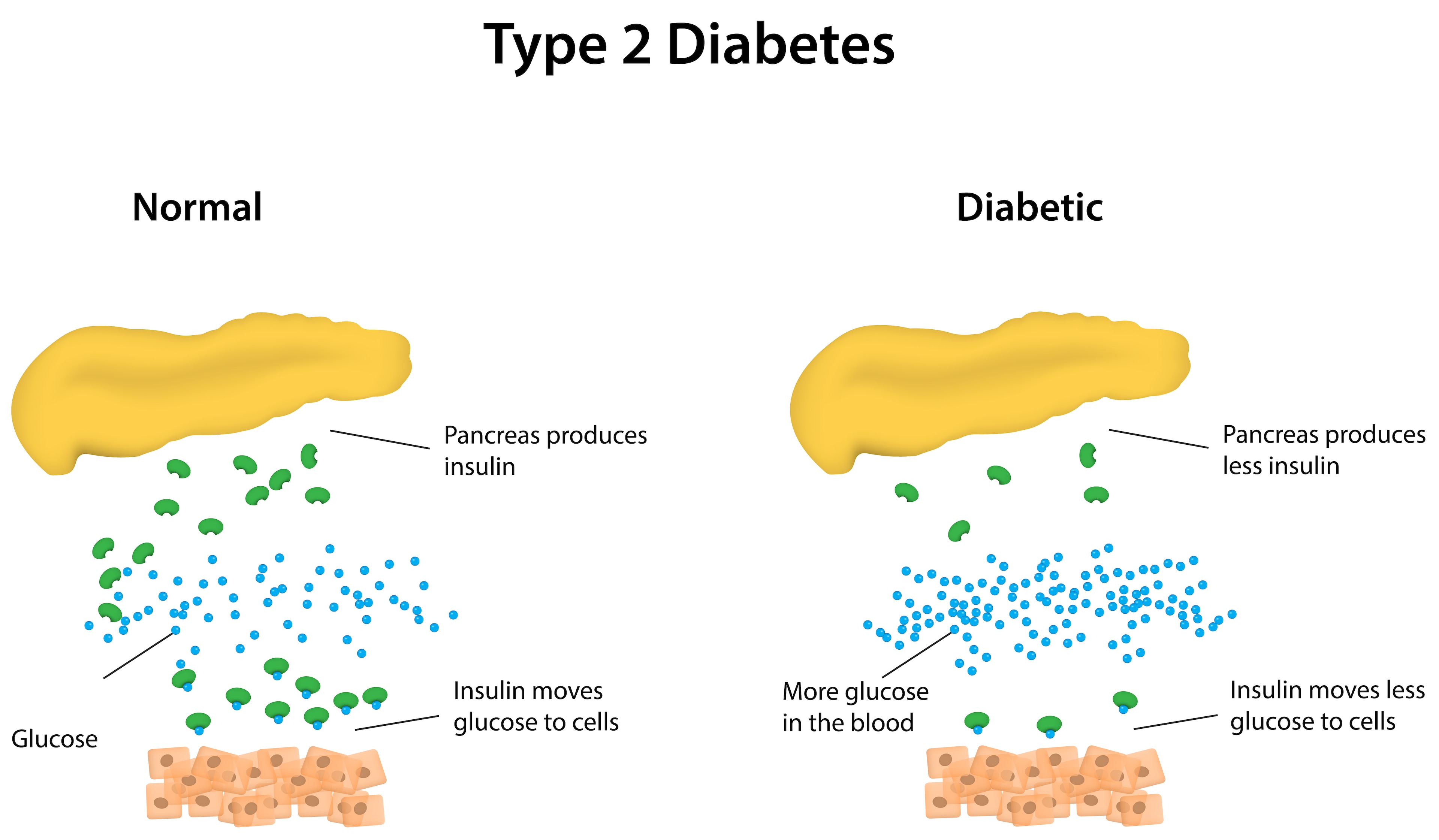
The intriguing question of why this happens remains unanswered.
Still, it opens the door to a potential marker for diabetes severity and the prospect of tailoring care based on each patient’s unique chronotype.
The implications extend beyond diabetes.
The timing of sample collection, whether morning or evening, plays a crucial role, impacting the accuracy of results. This phenomenon echoes in clinical settings, where the timing of examinations or the administration of treatments at different hours can influence diagnoses and the efficacy of therapeutic interventions.
Unravelling the LysoPI Enigma
Charna Dibner and Pierre Maechler expanded their lipidomic investigations beyond individuals with type 2 diabetes, encompassing a mouse model of pre-diabetes and patients who had undergone surgery, resulting in a substantial loss of insulin-producing beta cells—approximately half of them.
Their findings revealed a noteworthy correlation: a specific type of lipid, lysoPIs, exhibited an increase in instances where there was a sharp decline in functional β cells, even before the clinical symptoms of diabetes manifested.
In a subsequent experiment, the researchers administered lysoPI to diabetic mice, observing a notable boost in insulin production.
This phenomenon extended to in vitro testing on pancreatic cells derived from diabetic patients. Essentially, lysoPIs showcased their capacity to enhance insulin secretion, acting as a support mechanism when the number of beta cells decreases or when these cells encounter functional challenges. Intriguingly, lysoPI precursors are naturally present in certain foods, such as legumes.
The revelation of lysoPIs’ unexpected role opens up new avenues for exploration. It suggests the potential development of dietary supplements or molecules tailored to lysoPI receptors, offering a compelling strategy for diabetes management.
Additionally, considering the chronobiological profiles of patients could provide valuable insights into more personalised and practical approaches to diabetes care.












Comments 1