Are Diamonds actually raining down?
We are not kidding; it is, indeed.
A recently conducted experiment suggests that hundreds of exoplanets may experience diamond rain in space. The origin of these diamonds lies in the highly compressed carbon compounds found deep within the core of exoplanets, operating at extremely low temperatures.
The ice giants of the solar system
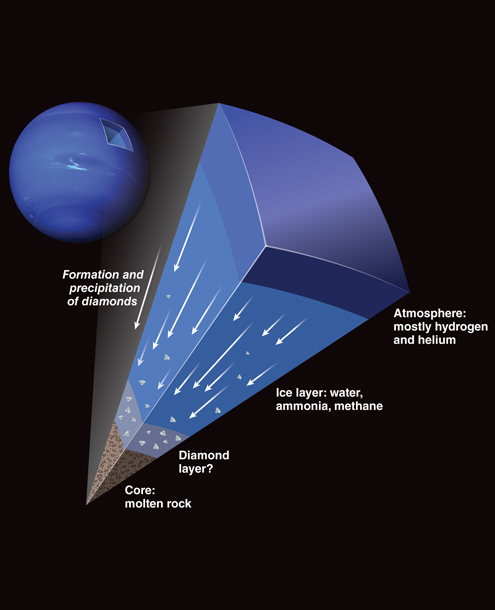
Known as the “ice giants” of our Solar System, Neptune and Uranus derive this title from the composition of their outer layers, which primarily consist of compounds containing hydrogen and helium.

In astronomical terminology, “ice” encompasses all compounds of light elements that incorporate hydrogen. Consequently, the presence of water (H2O), ammonia (NH3), and methane (CH4) in these planets planets atmospheres classifies them as “icy” celestial bodies.
The distinctive and captivating bluish tint exhibited by both Neptune and Uranus can be attributed to the traces of methane within their atmospheres.
Is it possible for diamonds to be present on the ‘ice giants’?
Under the extreme heat and pressures within the ice layers of Uranus and Neptune, ammonia and methane exhibit chemical reactivity. Scientists have undertaken modelling efforts to understand the exotic processes occurring deep within these ice layers, including the formation of diamonds.
The concept of diamond rain within these planets was first proposed by Marvin Ross of the Lawrence Livermore National Laboratory in a 1981 article titled “The Ice Layer of Uranus and Neptune—Diamonds in the Sky?”
According to this hypothesis, carbon and hydrogen atoms from hydrocarbons like methane separate under the intense pressures and temperatures within the ice giants. These isolated carbon atoms would then aggregate into a diamond structure, the most stable form of carbon under such conditions.
Due to its higher density than methane, ammonia, and water in the ice layer, the carbon crystal would start sinking toward the planets’ planet cores. As it descends, it accumulates new layers by interacting with other isolated carbon atoms or diamonds. This process allows individual diamond blocks to grow to meters in diameter.
As a result, a substantial layer of carbon is believed to surround the rocky cores of Uranus and Neptune. This carbon layer may exist as solid diamond blocks, or under extreme temperatures; it could transform into liquid carbon or a combination of solid and liquid carbon.
If the carbon layer is a solid and liquid carbon mix, the stable carbon would have a lower density than the liquid form. This could lead to the intriguing possibility of large ” diamond bergs” floating on top of an ocean of liquid carbon.
The distinct composition of the carbon layer—whether solid, liquid, or a combination—would have varying effects on the planetary core.
How was the study conducted?
Conducted at the SLAC National Accelerator Laboratory in California, the experiment, led by Mungo Frost and his team, indicates that diamond rain could emerge as a prevalent occurrence within ice-giant planets such as Uranus and Neptune. Frost’s and Frost’s findings shed light on the potential regularity of this extraordinary phenomenon within the atmospheric realms of these distant celestial bodies.

In previous studies, numerous laboratory experiments have investigated the circumstances conducive to diamond formation within ice giants. The majority of these experiments employed a dynamic compression method. However, in a unique move, the recent experiment represents the first instance where the static compression method was used.
What were the findings?
The investigation into the conditions of compressed carbon on icy planets involved a unique approach, utilising static compression coupled with dynamic heating during the experiment.
Mungo Frost and his team experimented by subjecting polystyrene, the polymer commonly used in styrofoam, to static compression. The process involved compressing polystyrene between two diamonds and bombarding it with X-ray light pulses. The outcomes of the experiment left the researchers astounded.
Surprisingly, the team observed a gradual formation of diamonds from the polystyrene under conditions of approximately 2200 degrees Celsius and pressures reaching around 19 gigapascals. These observed conditions closely mimic those found within the interiors of ice giants like Uranus and Neptune, providing crucial insights into the potential for diamond precipitation within these distant planets.
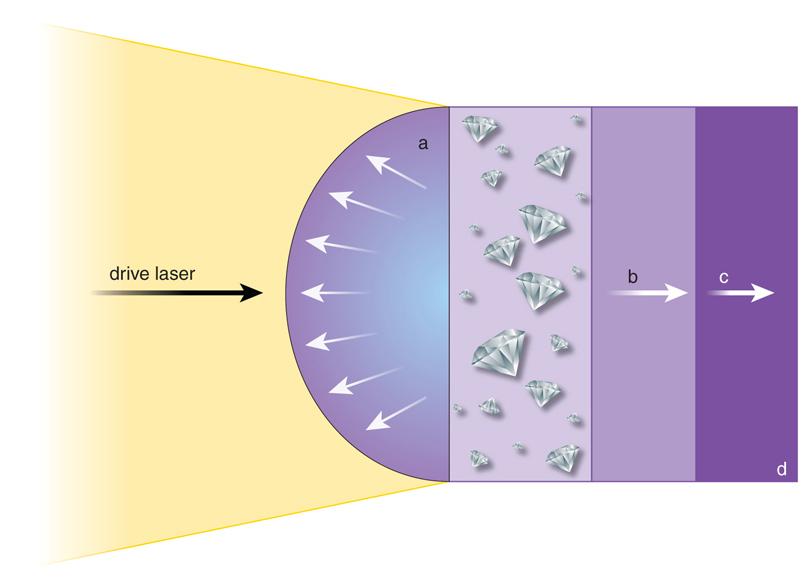
The pressures observed in this experiment were notably lower than those previously deemed essential for diamond formation in dynamic compression experiments. Additionally, the reaction duration surpassed that of dynamic compression experiments, providing a potential explanation for why low-pressure diamond formation hadn’t been detected in prior studies.
“It disagreed with established results and wasn’t what we expected to see, but it fits in nicely and sort of tied everything together,” expresses Frost. “It turns out that was all down to different timescales.”
Subsequently, the research team concluded that the prospect of diamond rain extends to numerous more minor planets. Out of the 5600 confirmed exoplanets, the researchers identified over 1900 as potential candidates for experiencing diamond precipitation.
Why is this understanding critical?
This revelation suggests a paradigm shift in our understanding of the formation of diamonds within solar systems, hinting that these precious gems might originate at shallower depths than previously envisioned.
The implications of this discovery also extend to altering our perceptions of the internal compositions of giant planets, opening new avenues for exploring the intricate dynamics at play within these celestial bodies.











