Overview
In a noteworthy stride towards battling dengue fever, Johnson & Johnson has achieved a remarkable breakthrough by successfully developing an experimental pill. Encouraging outcomes have emerged from a preliminary human challenge trial, marking a significant advancement in the ongoing battle against the disease.
Dengue: The Global Threat
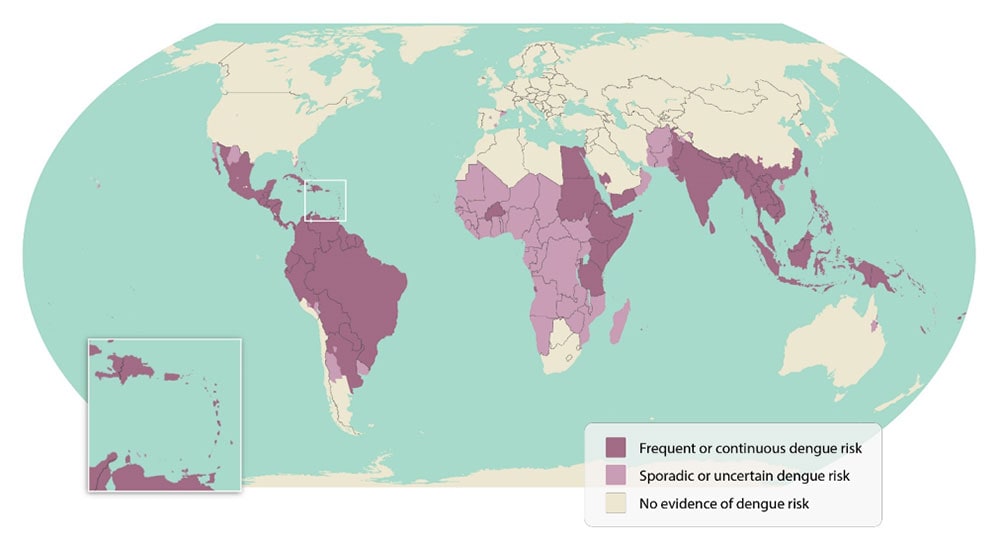
Dengue also known as breakbone fever or dandy fever, is a viral illness transmitted by Aedes mosquitoes, is prevalent in tropical and subtropical areas. These mosquitoes breed in the surroundings of human dwellings. While most cases manifest as feverish illnesses, severe cases such as hemorrhagic fevers and shock occasionally lead to fatalities.
As per the World Health Organization, dengue fever stands among the top ten global health threats, and it is the fastest-spreading among them. The past half-century has witnessed a staggering 30-fold surge in global incidence.


With over half of the world’s population at risk, approximately 390 million dengue infections occur annually, leading to a concerning toll of up to 36,000 deaths. Recent years have witnessed the most substantial dengue epidemics in Southeast Asia, the Americas, and the Western Pacific regions. The impact is widespread, with around 141 countries grappling with the effects of this infectious disease.
The absence of a specific medical treatment for dengue has posed a significant challenge in managing the infection. Currently, there is no dedicated medicine tailored for breakbone fever treatment. This underscores the importance of breakthroughs such as Johnson & Johnson’s experimental pill, representing a potential game-changer in the fight against the breakbone fever. Developing a specific treatment can significantly enhance our ability to address the disease’s impact and improve outcomes for those affected.
All you need to know about the pill.
In a groundbreaking development, pharmaceutical giant Johnson & Johnson has unveiled early data suggesting that its antiviral pill, JNJ-1802, exhibited shielding effects against dengue in a clinical trial.
The study, presented at the American Society of Tropical Medicine & Hygiene Annual Meeting in Chicago, involved healthy volunteers who took either the experimental drug or a placebo for 26 days. Participants were injected with a dengue virus on the fifth day in a challenge trial setup.
Over an 85-day monitoring period, the participants were assessed for signs of dengue infection, including immune responses and the presence of the virus in their blood.
Johnson & Johnson reported that the JNJ-1802 drug was well-tolerated and safe for all participants. Intriguingly, while all five individuals in the placebo group exhibited detectable virus levels, six out of ten participants who acquired a high dose of the experimental drug showed no signs of infection.
Although these premature results are deemed “promising,” it is underscored that further, larger-scale tests are needed for confirmation.
Marnix Van Loock, head of emerging pathogens research at Johnson & Johnson’s Janssen branch, expressed positiveness, remarking that these findings provide hope for scientific progress in addressing the escalating global threat posed by dengue. This expansive trial traverses ten countries, including high-risk regions like the Philippines, Thailand, Peru, Brazil, and Colombia.
In distinction to controlled challenges where a weakened dengue virus is used, this real-world trial aims to evaluate the drug’s effectiveness in preventing the dandy fever across all four circulating strains.
How does the pill work?
Johnson & Johnson has revealed that the mechanism of action for its drug involves deterring viral proteins, effectively arresting the replication of the dengue virus.
While the proceeding trial predominantly evaluates the drug’s preventative capabilities, its demonstrated antiviral activity suggests possible applicability as a treatment for those already afflicted.
The company has sketched intentions to further investigate the drug’s therapeutic potential in succeeding trials, marking a noteworthy stride towards addressing dandy fever infections comprehensively.
The potential significance of this development cannot be overstated. If ascertained effective on a larger scale, this antiviral pill could revolutionise the approach to dengue, offering both prevention and treatment options. We can only stay hopeful that this pill will bring us to a future where the impact of the breakbone fever is massively reduced, if not eradicated.












Comments 1