CAR-T Approval in India: A milestone in healthcare
Introduction
In the radiant landscape of medical innovation, where breakthroughs often resemble flashes of brilliance, ImmunoACT emerges as a shining beacon.
Born from the esteemed halls of IIT Bombay and nurtured within the Society for Innovation and Entrepreneurship (SINE) cocoon, ImmunoACT is a testament to the potential harnessed when academia and entrepreneurship intertwine.
This IIT Bombay spin-off has engraved its mark in history as a pioneer, achieving a remarkable milestone –regulatory approval for India’s inaugural CAR-T cell therapy by the Central Drugs Standard Control Organisation (CDSCO).
In a country where healthcare strides are as critical as they are transformative, ImmunoACT’s journey from ideation to regulatory recognition is not just a triumph for the startup but a leap forward for the entire Indian healthcare ecosystem.
The Backdrop of CAR-T
Amidst India’s stark reality of ranking second in global cancer mortality, a formidable challenge arises as 40% of patients prove refractory to conventional therapies, particularly in Leukaemia and Lymphoma cases.
However, a glimmer of hope emerges through CAR-T cell therapy, boasting an 80% response rate—significantly surpassing the efficacy of chemotherapy, biologics, and bone marrow transplants.
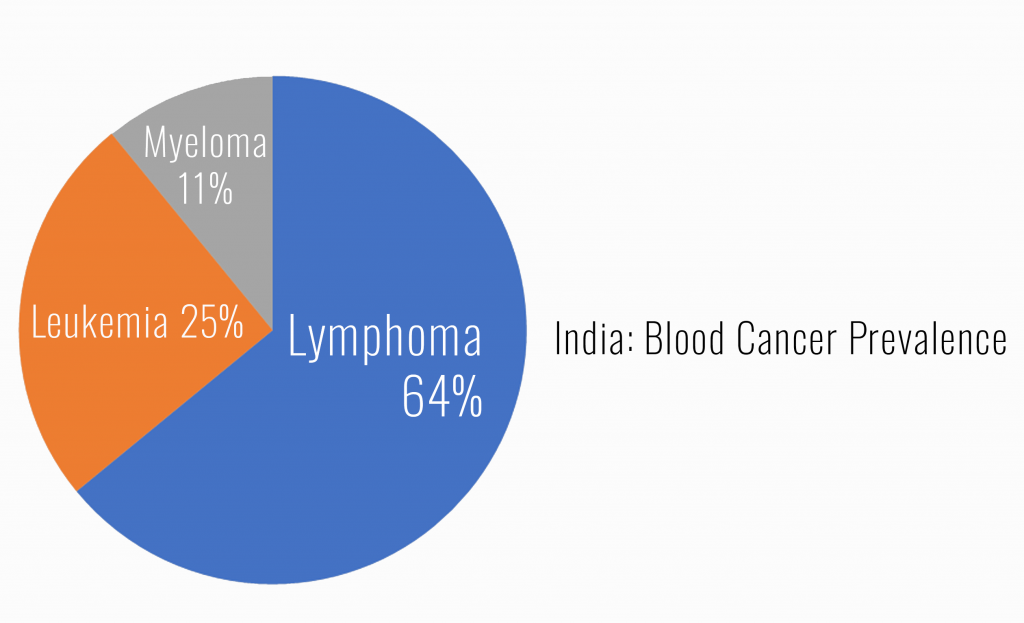
In a landscape rife with high mortality rates and treatment complexities, CAR-T cells present a revolutionary avenue, promising a potent and highly effective alternative for patients facing relapse or resistance to traditional cancer treatments.
CAR-T Cell therapy
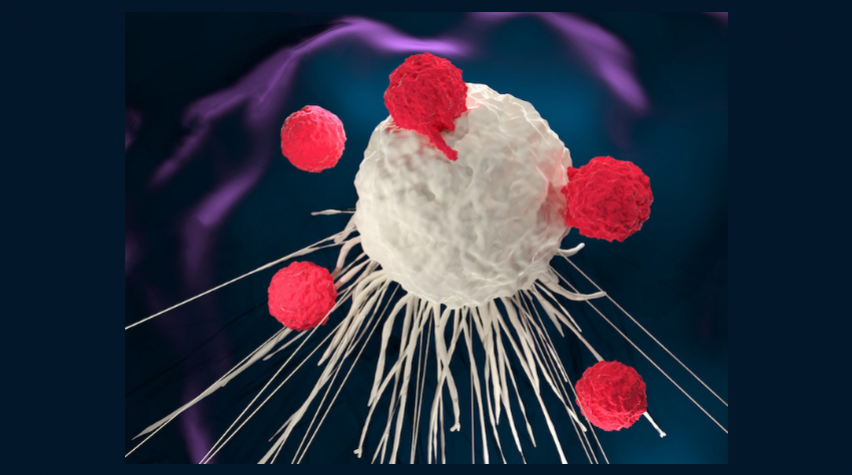
At the forefront of groundbreaking advancements in blood cancer treatment lies chimeric antigen receptor (CAR)-T cell therapy, heralded as one of the latest and most promising interventions. This innovative technique harnesses the body’s immune system to combat cancer, representing a paradigm shift in therapeutic strategies.

Penn Medicine, a trailblazer in the early exploration of CAR T cell medications, remains at the vanguard, pushing the boundaries of research to expand the repertoire of CAR T cell drugs and enhance their efficacy.
What is CAR-T therapy?
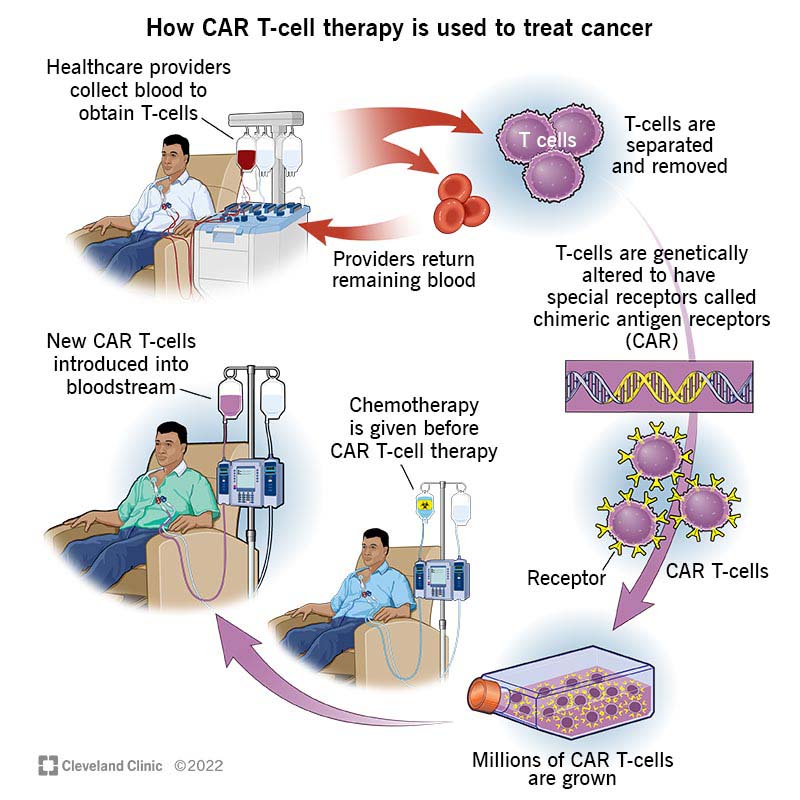
CAR T-cells, often called “living drugs,” are unique because they stay inside the body long.
To make them, scientists take cells from the patient’s blood, modify them using a harmless virus, and turn them into smart cells called CARs. These smart cells learn to recognise and destroy cancer cells.
After this process, these engineered cells are put back into the patient’s blood, where they continue to fight against cancer. It’s like giving the body its superhero cells to battle the disease.
Why CAR-T therapy?
CAR-T cell therapy stands as a revolutionary frontier in cancer immunotherapy, utilising genetically modified T-cells to empower them to identify and eliminate cancer cells precisely.
This approach, honed in laboratory settings, enhances the effectiveness of T-cells, providing a potent weapon against malignancies. Its power becomes particularly evident when traditional treatments fail, making CAR-T therapy a beacon of hope for patients facing resilient cancers.
CAR-T therapy’s impact extends to several types of haematological malignancies in treating leukaemia, lymphoma, and multiple myeloma.
This regulatory endorsement underscores the therapeutic potential of CAR T cell therapy in addressing a diverse array of challenging cancers within the haematological domain.
Consequently, patients grappling with these conditions have renewed optimism, as CAR-T therapy represents a strong alternative when conventional treatments prove poor.
Why is the approval of CAR-T therapy in India significant?
Introduced in 2017, CAR T-cell technology offers promising prospects for cancer treatment.
Unfortunately, this advanced therapy is unavailable in India and costs millions abroad. However, a private company plans to import the technology, making it accessible to Indian patients at around ₹35,00,000.
Professor Rahul Purwar and his team from the Department of Biosciences and Bioengineering, who have developed this breakthrough, promise effective treatment and tackle the cost issue.
While imported treatments may demand crores, the indigenous approach aims for a more affordable single-dose treatment at approximately ₹15 lakhs. Professor Purwar’s innovation could significantly bring cutting-edge medical solutions within reach for a more extensive section of society.
Conclusion
In conclusion, the approval of India’s first CAR-T cell therapy is a monumental achievement for healthcare and a giant leap for humanity and scientific innovation. ImmunoACT’s accomplishment symbolises a promising era where cutting-edge medical solutions become accessible. This is more than a breakthrough; it’s a beacon of progress where science converges with healthcare to redefine the future of medical possibilities.